Complete facilities to produce Products for Haemodialysis and Peritoneal Dialysis. In several cases these manufacturing lines are installed in devoted areas of Intravenous Solutions Projects.
DIALYSYS SOLUTIONS
Priming Solution
Haemodialisys Concentrate
Pre-mixed Heparin
DIALYSIS FORMS
- Canisters
- Bags
- Multichamber bags
- Cartridges
DIALYSIS PRODUCTS
- 3.4 / 5 L Diacetate bags
- 5 / 8 / 10 L Diacetate and Bicarbonate canisters
- CAPD bags
- Bicarbonate cartridges
- Priming bags
- Multichamber bags
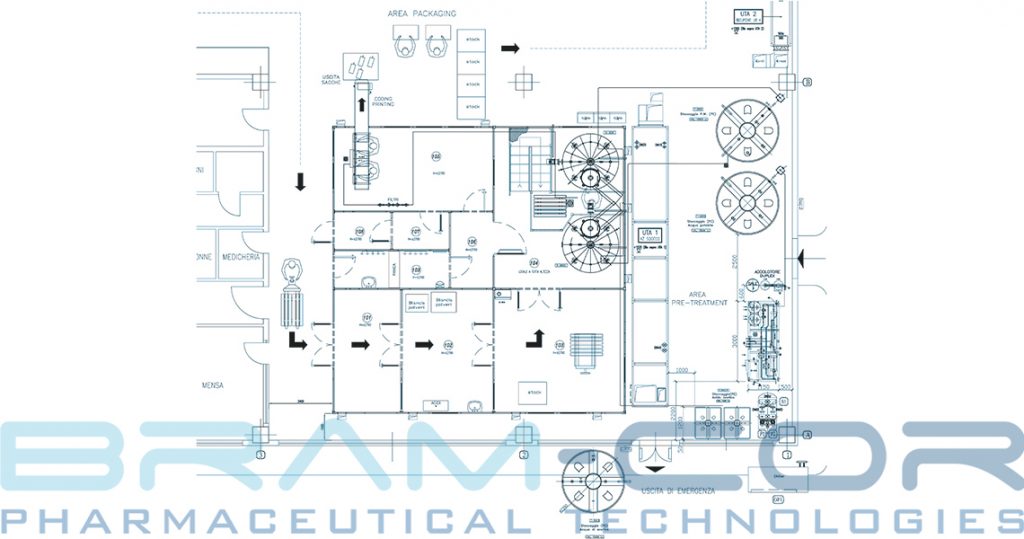
FROM DESIGN TO VALIDATION, WE ARE ALWAYS CLOSE TO THE CUSTOMER
The standards of good manufacturing practice (cGMP) require special attention to risk assessment and verification procedures: “… it is requirement of good manufacturing identify the activities of validation necessary to demonstrate control critical aspects of particular operations. The significant changes made to installations, equipment and processes, which may affect product quality, should be validated. A procedure for risk assessment should be used to determine the scope and extent of validation.” The Validation Master Plan serves to make sure that all equipment, procedures, that may affect the quality or integrity or effectiveness of the product, are validated; it contains the general principles which comply during the validation task, and plans activities to be carried out for this purpose.

BRAM-COR TURNKEY FOR DIALYSIS PRODUCTS / STANDARD STEPS
√ Basic Engineering √ Detailed Engineering √ Design Qualification √ Inlet Water Pretreatment Plant √ Pharmaceutical Water Systems (Softened, Purified and Distilled Water) √ Pharmaceutical Processing and Solution Preparation Systems √ Pharmaceutical Forming, Filling, Inspecting, Packaging lines √ Clean Rooms √ Epoxy coating of the floors √ HVAC and air treatment plant √ Autoclave √ Pure Steam Generator and PS circuit √ Laboratories of Analysis (Microbiological / Chemical) √ Site Master Plan √ Validation Master Plan √ Installation √ Training √ Start up √ Technical Files & Documentation √ IQ/OQ √ PQ Protocols √ Validation at Site √ Standard Operating Procedures √ Initial Know How Transfer √ GMP preAudit √ Spareparts for n years